Investigating a new electrical stimulation treatment for drug-resistant epilepsy
Why is a new treatment needed for drug-resistant epilepsy?
Epilepsy affects over 50 million people worldwide. While vagus nerve stimulation at neck level is proven to reduce seizures in drug-resistant patients, side effects from the stimulation can include coughing, voice alteration, headaches and worsening sleep apnoea.
Research at the Bionics Institute
In 2022, Bionics Institute researchers started investigating the effectiveness of stimulating the vagus nerve at abdominal level to treat epilepsy.
Our researchers have developed a small electrical device to deliver higher-intensity stimulation to the vagus nerve, which aims to improving seizure control without side effects.
Preliminary findings indicate that abdominal stimulation can activate the brain region essential for the therapeutic effect of neck level vagus nerve stimulation, without side effects on the larynx, lungs and heart.
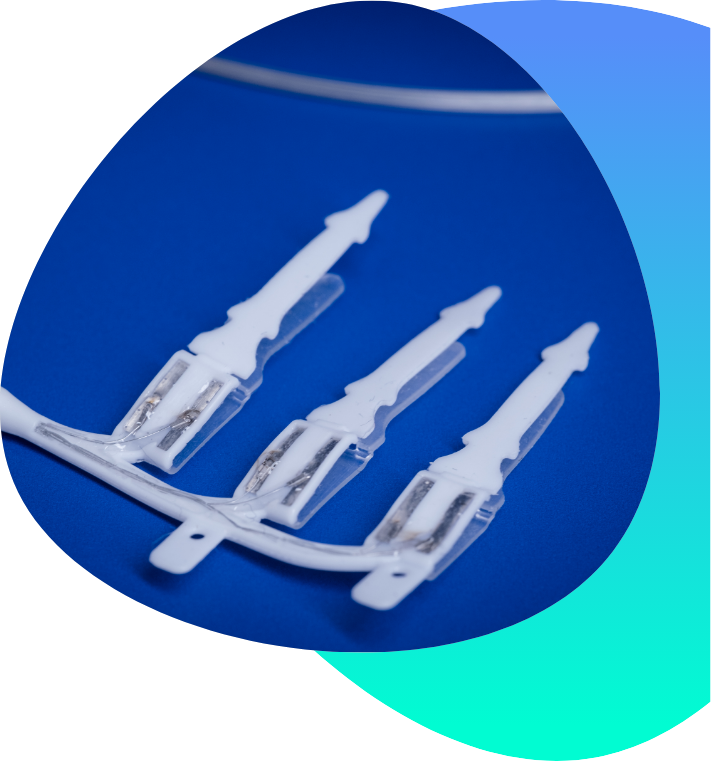
The device, made up of tiny electrodes encased in silicon, is attached to the vagus nerve like a clip during keyhole surgery – a 90-minute procedure.
Connected to a stimulator implanted under the skin at hip level, the device is powered by a battery that lasts for 10 years.
Next steps for Bionics Institute researchers
Our vagus nerve stimulation device was originally developed to treat inflammatory bowel disease, and is currently in clinical trial for treatment of Crohn’s disease
The clinical trial is called the ElectRx study (NCT05469607) and is currently recruiting patients at the Austin Hospital.
The safety data from this first clinical trial will support other applications of our vagus nerve stimulation technology in the future.
The next phase of our research involves evaluating the effectiveness of abdominal vagus nerve stimulation to treat epilepsy and moving towards clinical trials.
The research team
Bionics Institute researchers:
Dr Tomoko Hyakumura, Professor James Fallon, Dr. Sophie Payne
Publications
Hyakumura et al., 2024 Journal of Visualized Experiments. https://doi.org/10.3791/65896